Industries
May 2022
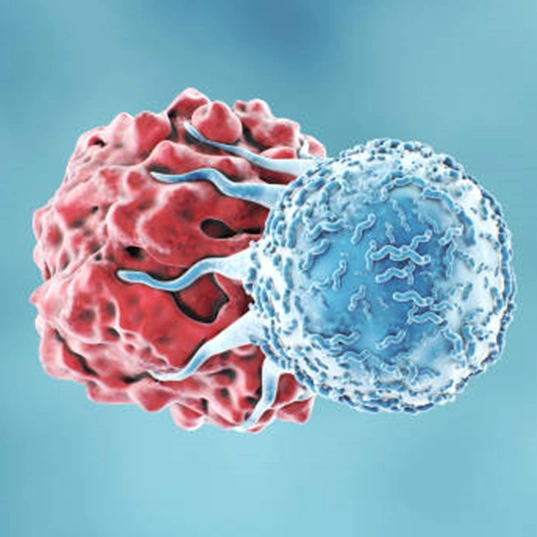
Precision medicine is one of the latest frontiers in modern medicine for patient treatment. It uses genetic information or other biomarkers from an individual to create a tailor-made treatment for that patient. Helbling is currently working with many companies on system design and implementation to democratize these treatments, such as CAR-T therapy (Figure 1), by automating laborious lab processes, improving biological quality control systems, and ultimately helping to move cell manufacturing from the lab to the patient’s bedside.
Recent years have seen an influx of new clinical trials based on cell engineering technologies. Old (viral/non-viral vectors) and new (CRISPR/Cas) cell transformation techniques enable more precise correction of genomic aberrations or the introduction of new characteristics in human cells while reducing the risk of off-target modifications. Personalized medicines such as CAR-T therapy are tailor-made for individual patients.
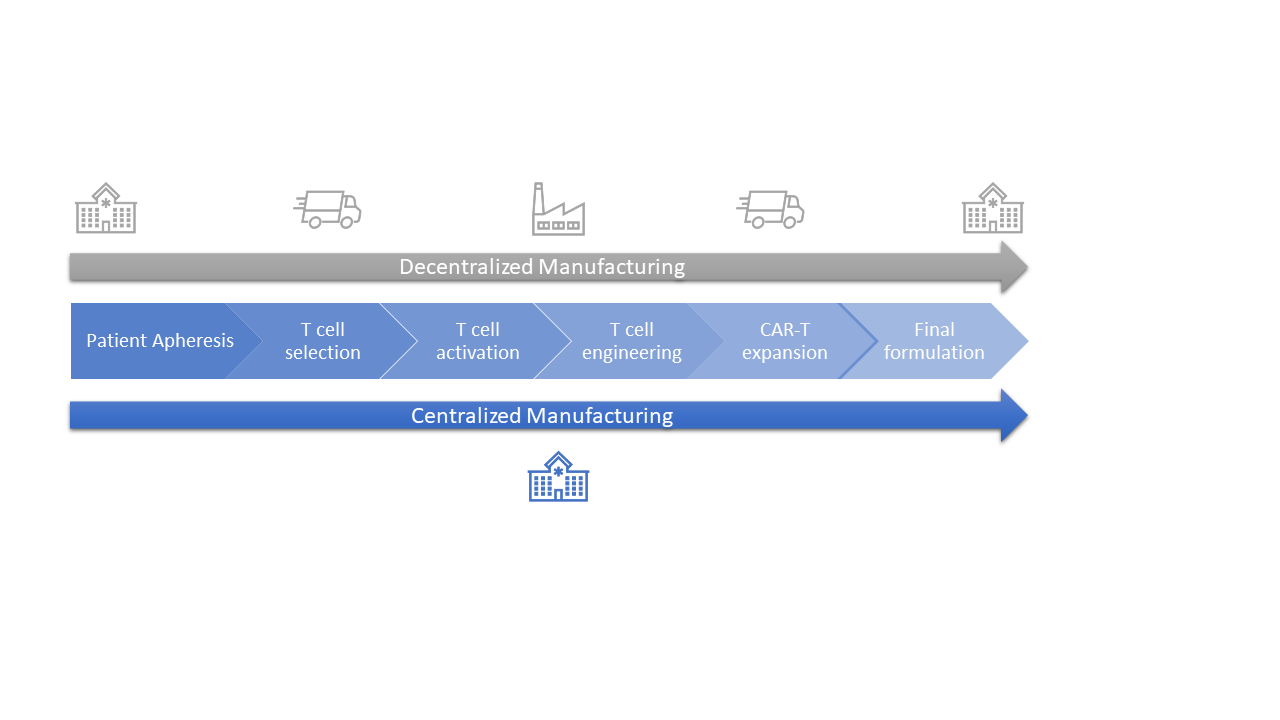
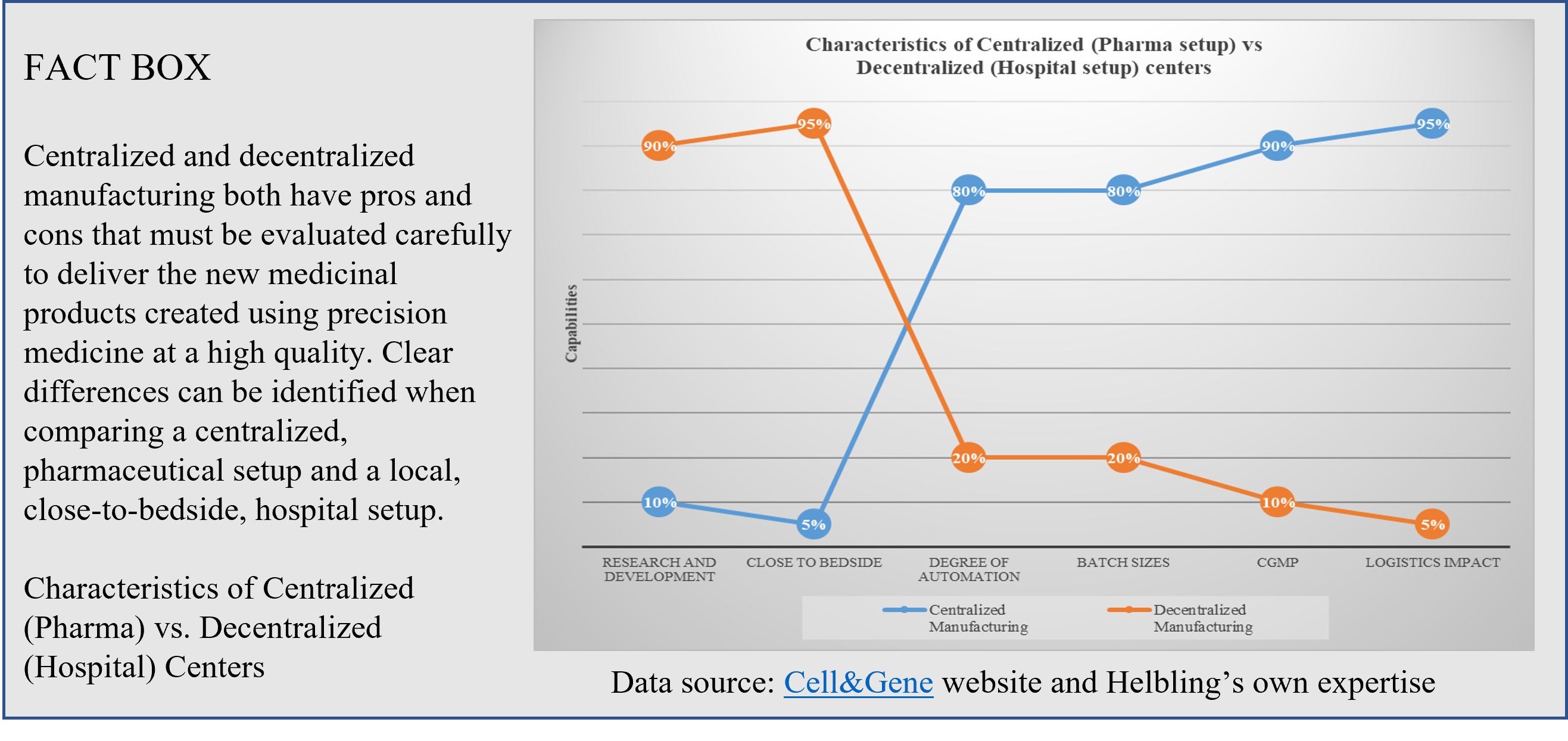
Chimeric antigen receptor T-cell (CAR-T) therapies against cancer cells are at the forefront of the portfolio of new drug products, with six autologous products having been approved by the US Food and Drug Administration (FDA)[1] and more than a hundred clinical trials currently running worldwide. Manufacturing CAR T-cell therapy products is a complex and lengthy process that typically takes more than two weeks. From cell collection to CAR T-cell infusion back to the patient, the process consists of numerous unit operations performed ex vivo, which requires fully trained and skilled operators, highly sterile environments, and labs that are compliant with current good manufacturing practices (cGMP). All of these steps have an impact on the success and cost of the final medicinal product. Therapy providers are currently working to democratize cell and gene therapies (CGT) and Helbling has worked on several such projects, developing specialized systems to enable the manufacturing process to be moved from centralized labs to the clinic, progressing first to hospital cGMP facilities, and then eventually to the patient’s bedside. This latter scenario is more important for autologous than for allogenic CAR-T therapies. Using centralized manufacturing labs away from the patient puts pressure on transportation, which has a high impact on costs. Decentralization of hospital processes requires a cGMP-compliant infrastructure for each CGT program, which entails administrative and operational costs (Figure 2).
Manufacturability of autologous CAR-T therapies
The uniqueness of every individual becomes clear when transplanting organs, tissues, or cells from one person to another. Using a patient’s own cells obtained by leukapheresis allows clinicians to apply CGT by avoiding or reducing the risk of rejections of the new medicinal product. That said, every patient is different and may be at various stages of cancer treatment, which has an impact on the quality and quantity of target cells available for CAR-T therapies.
The peculiarity of the starting material is a definitive challenge for process standardization and automation in cell manufacturing. Typically, novel CAR-T therapies are initially developed in academic or research laboratory environments, where ad-hoc therapies can be tailored on a patient-by-patient basis. Then, these early processes are transferred to internal or commercial partners to develop efficient cGMP manufacturing processes for clinical and commercial use. The variability of patient starting material and specific processing requirements do not allow for a one-size-fits-all approach, challenging the ultimate vision of a fully integrated system at the patient’s bedside.
Helbling is familiar with this challenge of building safe, sterile, fully integrated cell processing systems, having successfully completed several such projects for clients working to democratize these treatments. Development of these systems typically requires deep biological process know-how, methodical development of reliable control systems, integration of custom injection molded components, creative development of novel quality sensor systems, and methodical verification of system performance (both hardware and software).
T-cell selection
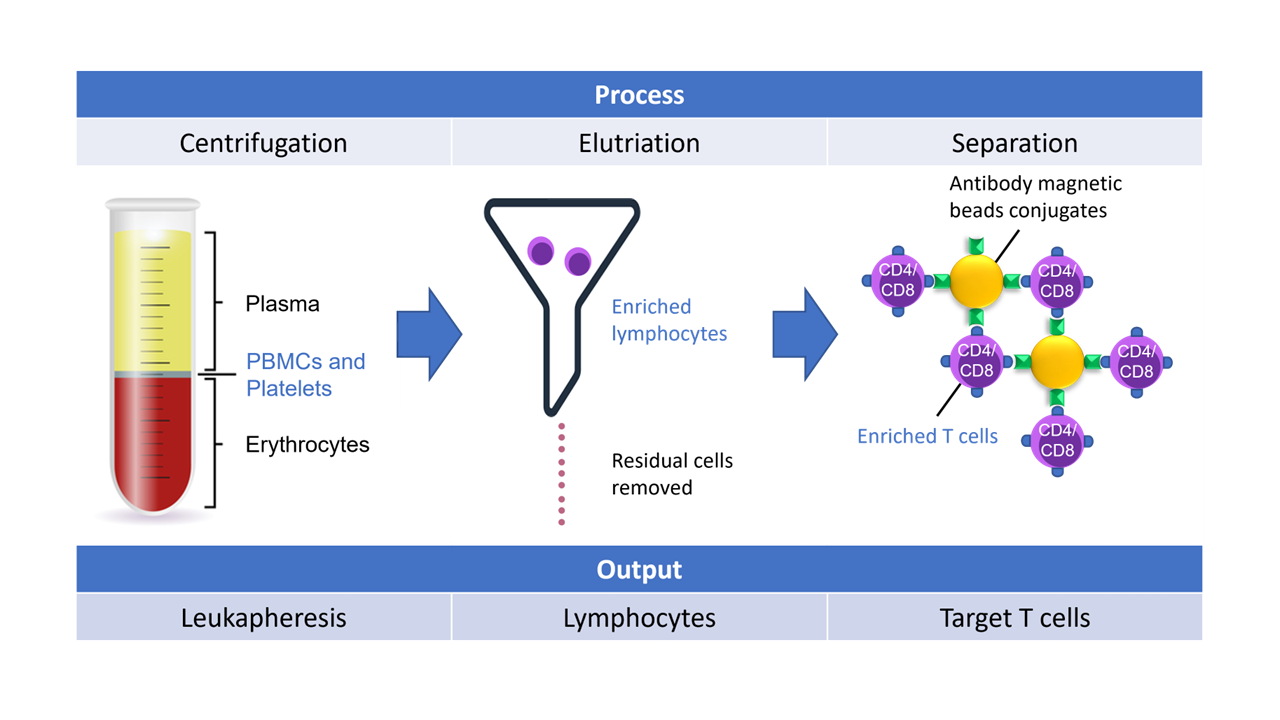
Starting with leukapheresis or whole blood collection bags, targeted separation of peripheral blood mononuclear cells (PBMCs) from red blood cells (RBCs) and other plasma components is critical for the CAR-T process as it allows for concentration of target cells only for downstream molecular engineering.
In one such systems project, Helbling was tasked with developing a purpose-built elutriation system to fractionate one component of whole blood, but at speeds not achievable with systems currently on the market. Helbling’s team used first principles, developing novel elutriation chambers, integrating single-use kits, coupling this into a valving and centrifuge system, and then implementing the software controls necessary to perform the process. The team has also designed, built, and tested similar systems using magnetic cell separation principles and centrifugation mechanisms (Figure 4).
T-cell activation and genetic modification
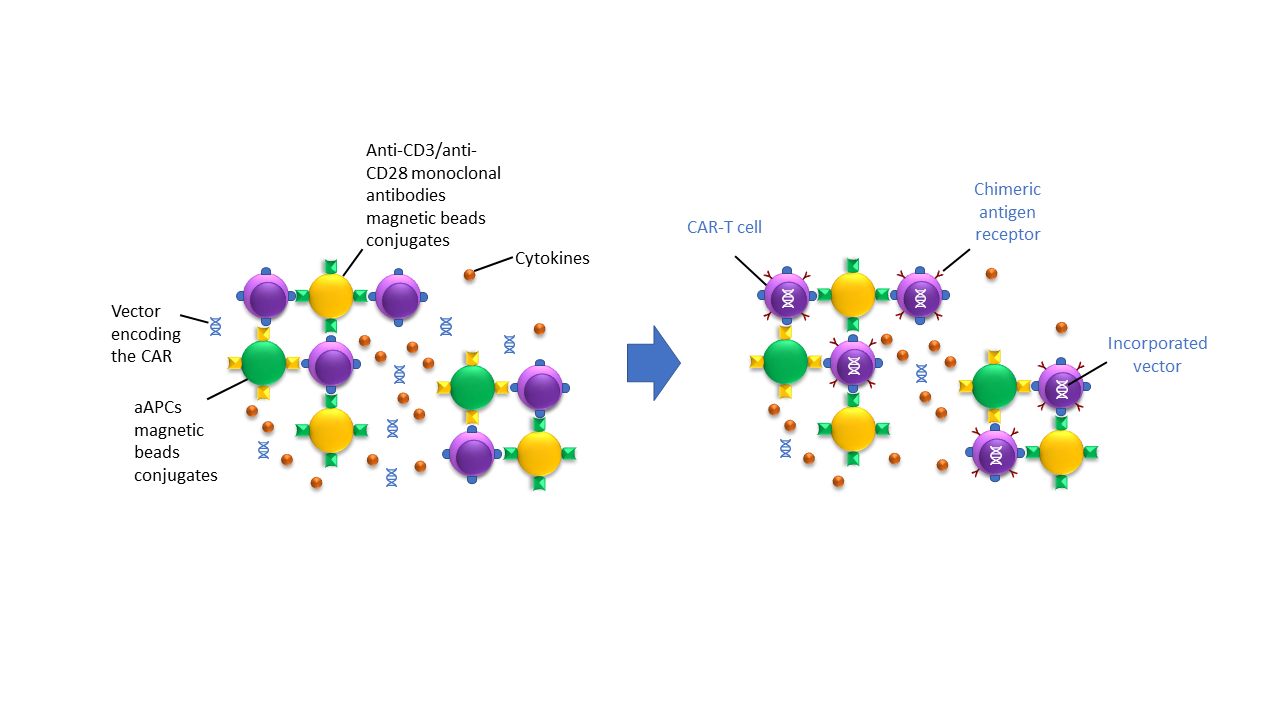
Following selection, targeted T-cells must be grown and stimulated ex vivo. This is done using magnetic beads coated with anti-CD3/anti-CD28 monoclonal antibodies or with cell-based artificial antigen-presenting cells (aAPCs) and growth factors like cytokines and interleukin 2 (IL-2). During the activation process, the T-cells are incubated with the viral/non-viral vectors, encoding the chimeric antigen receptor (CAR). Once incorporated, the CAR is then transcribed and translated by the patient’s cells and expressed on the cell surface, as depicted in Figure 5. The newly modified CAR T-cells can now specifically recognize the cancer target for which the CAR has been designed [2]. All the steps and components described here must be carefully handled along the process.
Helbling’s expertise in designing and implementing precision dispensing, quality control, and liquid handling systems allows for reliable control of the T-cell activation and modification process. From engineering reagents to customizing consumables and microfluidic design, Helbling’s team has the cross-functional skills to optimize the cell transformation process for different methods (electrical, physical, or chemical membrane poration).
CAR T-cell expansion and final formulation
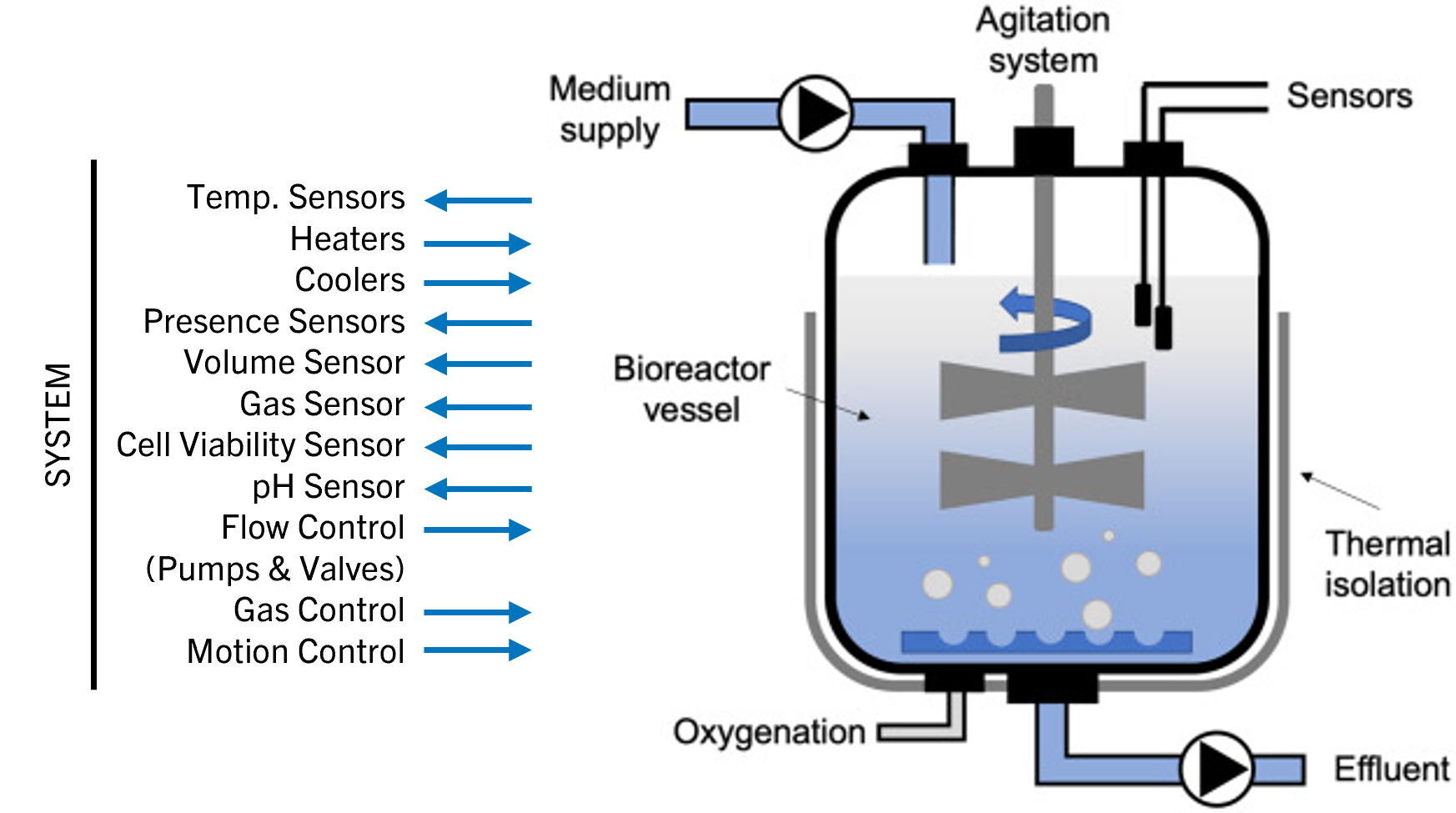
To achieve full potency for clinical use, a bioreactor culture system is used to grow the CAR T‑cells in large numbers. Optimal gas exchange, nutrient levels, temperature, and pH must be ensured, while toxic metabolites are removed and growth media exchanged by a semiautomated or completely automated system over several days (Figure 6).
Helbling was engaged in a project for the development of a fully contained GMP bioreactor for cultivating artificial (allogenic) scaffolds. The team was asked to design solutions that reliably control all needed liquid and gas exchanges along the process. This ensured full environmental control around the growing cells. Leveraging more than 25 years of experience in the medtech and biotech sector, Helbling can design and integrate sensors and quality control devices (such as flow cytometry) for in-line or at-line control of the most critical parameters to be monitored during cell expansion and collection.
Summary: Helbling Technik as an experienced partner in the development of novel cell and gene therapies
Like other cell and gene therapy techniques, CAR T-cell therapies promise to greatly expand treatments for cancers and other unmet pathological conditions, yet the high level of customization from batch to batch is challenging for consistent, high-quality production, which favors homogenous, unchanging processes.
That said, Helbling is meeting this challenge, together with its clients, by bringing diligent, tech‑savvy, creative systems engineering approaches to cell and gene therapy projects. The Helbling team has integrated and designed devices, systems, and solutions at every step of the process with operators and the cGMP environment in mind by leveraging a well-established (and audited) quality management system.
Helbling an ideal partner for developing systems, devices, and machines that enable new CAR T-cell therapy solutions.
References:
- CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. From the NIH website: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- T-cell selection from whole blood to enriched T-cells. Levine et al. Mol Ther Methods Clin Dev. 2016 Dec 31;4:92-101. doi: 10.1016/j.omtm.2016.12.006
Authors: Alessandro Fammartino, Cole Constantineau, Urs Anliker, Christian Peterhans