Project
DensiProbeTM – Torque Measurement System for Medical Applications in the Hip and Spine Region

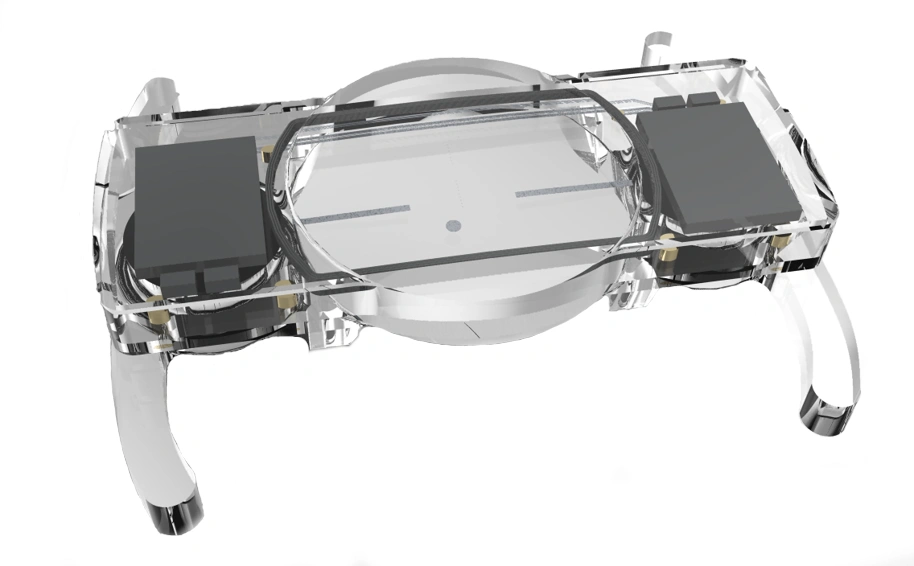
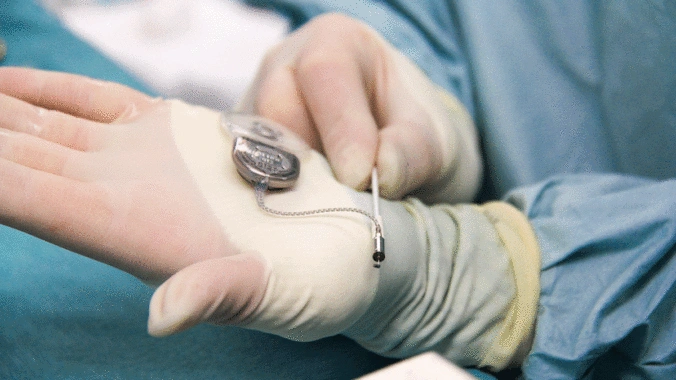
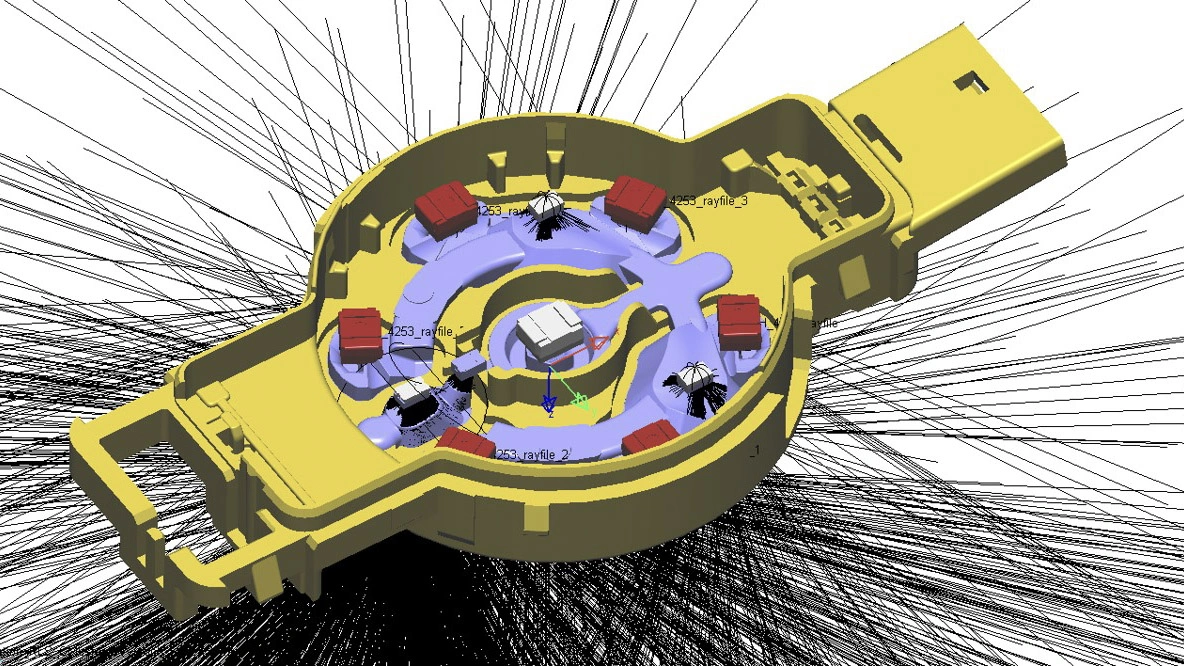
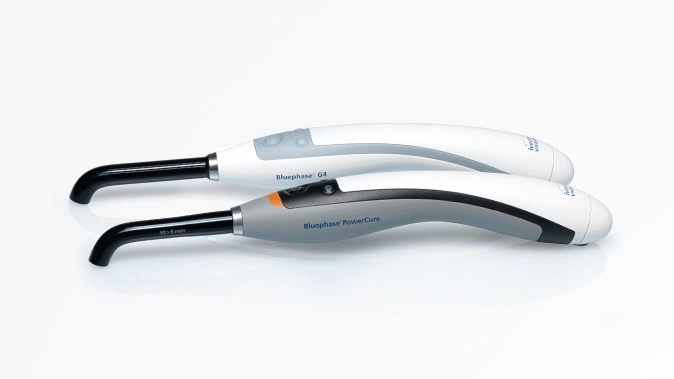
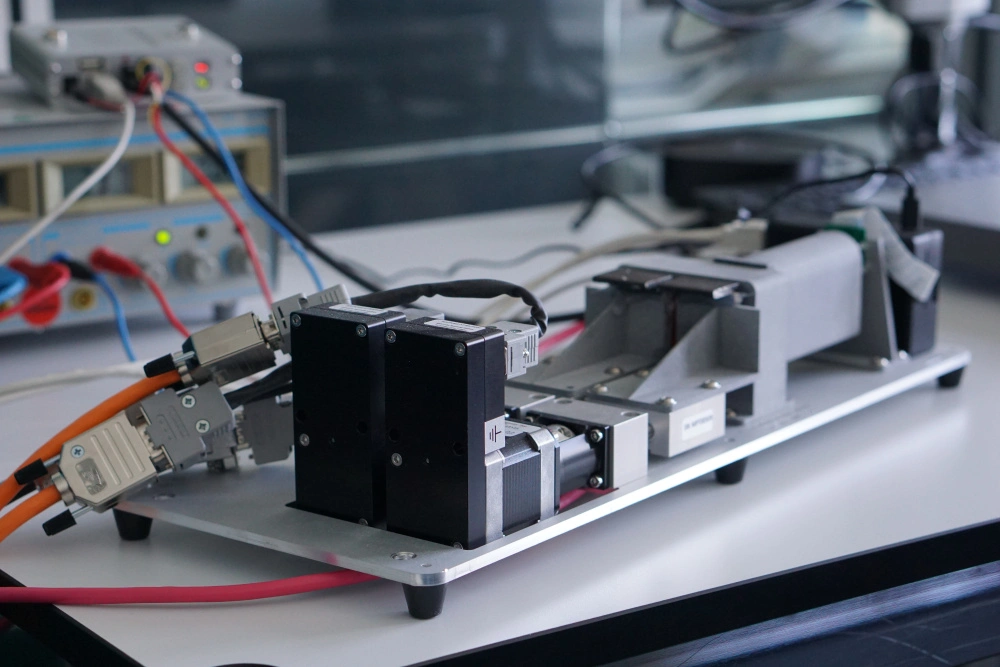
Surgeons need information about bone quality in order to provide the best possible treatment for femoral neck or vertebral fractures. In the past, they have relied on computed tomography (CT) scans performed prior to the surgical procedure. This kind of imaging, however, only gives them a rough estimation of bone quality and, in particular, density. DensiProbeTM gives them a tool that they can use to obtain precise bone density information during an operation by measuring breakthrough torque. Helbling developed DensiProbeTM on behalf of a respected research institute.
Bone quality assessments have been a concern of the medical technology industry for some time. If a patient's bone quality is in the critical range, for instance, the surgeon needs to adapt the procedure accordingly by selecting more suitable screw and plate systems. This is particularly true in the case of older patients who frequently suffer from osteoporosis (bone loss). In the past, however, bone density could only be assessed preoperatively with a CT scan. Hence a renowned private research institute decided to develop an instrument that would allow surgeons to perform intraoperative bone density measurements. The institute had the required medical expertise to carry out the conceptual design work for the DensiProbeTM as a system, and was focusing its resources on the interfaces to users (surgeons) and conducting clinical studies. On the strength of its proven expertise in the areas of embedded systems, low-power electronics, battery management, measurement technology and USB communication, Helbling was awarded the contract for developing the technical aspects of the overall measurement module. The biggest challenge was that the instrument should be battery-operated and easy to handle. On top of that, Helbling needed to observe all of the regulatory requirements of the EU Medical Device Directive and ISO 13485.
Bone quality assessments have been a concern of the medical technology industry for some time. If a patient's bone quality is in the critical range, for instance, the surgeon needs to adapt the procedure accordingly by selecting more suitable screw and plate systems. This is particularly true in the case of older patients who frequently suffer from osteoporosis (bone loss). In the past, however, bone density could only be assessed preoperatively with a CT scan. Hence a renowned private research institute decided to develop an instrument that would allow surgeons to perform intraoperative bone density measurements. The institute had the required medical expertise to carry out the conceptual design work for the DensiProbeTM as a system, and was focusing its resources on the interfaces to users (surgeons) and conducting clinical studies. On the strength of its proven expertise in the areas of embedded systems, low-power electronics, battery management, measurement technology and USB communication, Helbling was awarded the contract for developing the technical aspects of the overall measurement module. The biggest challenge was that the instrument should be battery-operated and easy to handle. On top of that, Helbling needed to observe all of the regulatory requirements of the EU Medical Device Directive and ISO 13485.
- Use as an operating room instrument
- Highly sensitive strain-gauge measurement method
- Battery-powered
- Low-energy embedded system
- Data logging, USB data readout
- Integrated in a mechanical system which can be sterilized
Services
- Technical definition of the medical device and production of the list of requirements (requirements engineering)
- Producing a functional model to demonstrate technical feasibility
- Producing energy management, circuit and PCB design in accordance with risk management
- Integrating and implementing embedded software
- Assisting the customer with system support during the phase of intended use testingComprehensive and compliant documentation throughout the development process in accordance with EU Medical Device Director and ISO 13485
Comprehensive and compliant documentation throughout the development process in accordance with EU Medical Device Director and ISO 13485
Result / Success
Several independent studies demonstrated that DensiProbe™ provides reliable data for bone density assessments in the case of femoral neck fractures.
Intraoperative bone density measurements performed with DensiProbe™ are more precise and economical than preoperative measurements obtained through a CT scan.
In the meantime, many positive articles have been published about DensiProbe™.
A medtech company is looking into the commercialization of the device.
Contact

Cases
Internationales Unternehmen der Papierindustrie – Analyse des Dampfsystems
Industries:
Services:
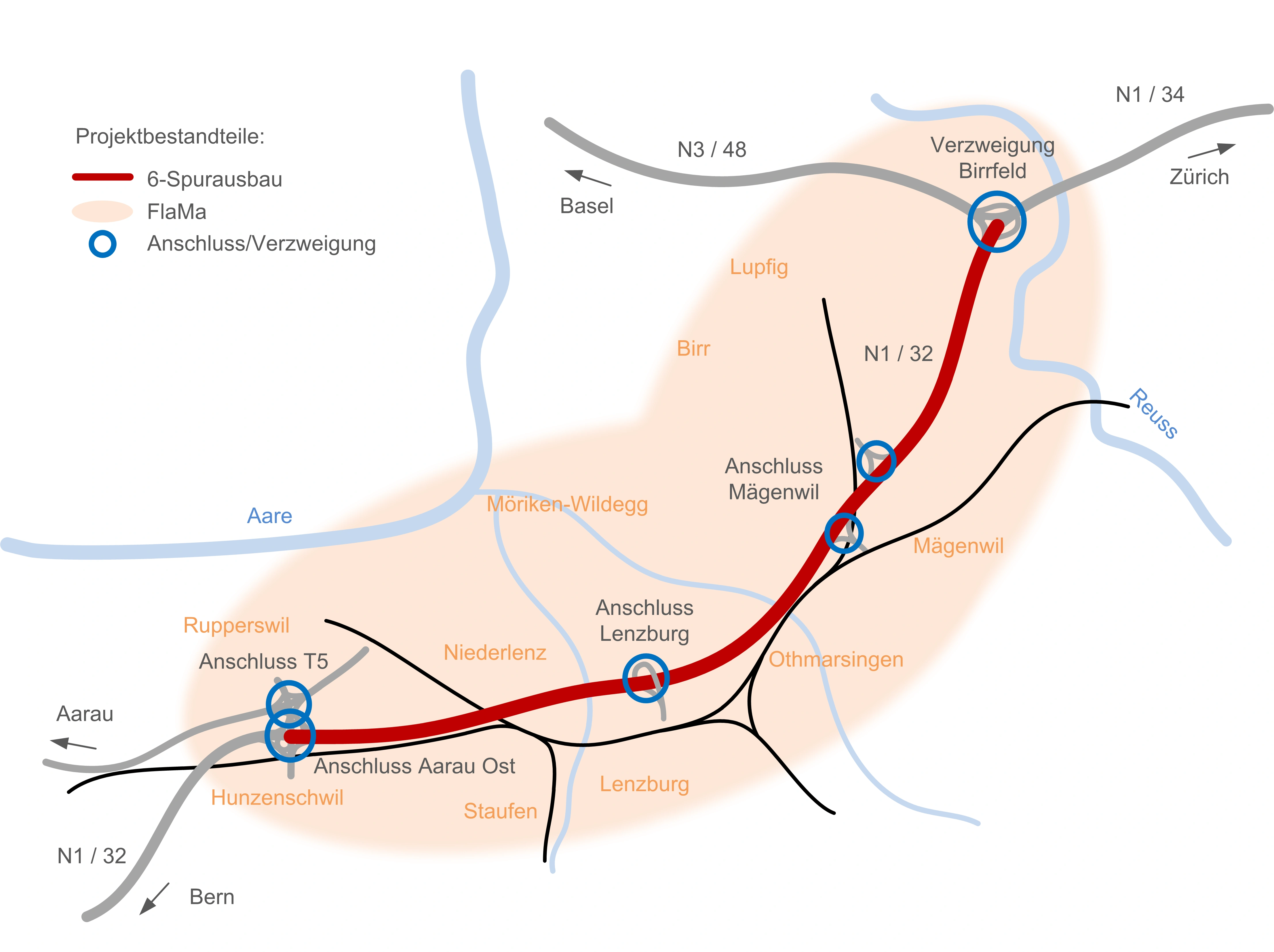
Cases
6-Streifenausbau Aarau Ost – Verzweigung Birrfeld – Bauherrenunterstützung
Industries:
Services: